Comprehensive Support
From Preclinical to Commercialization
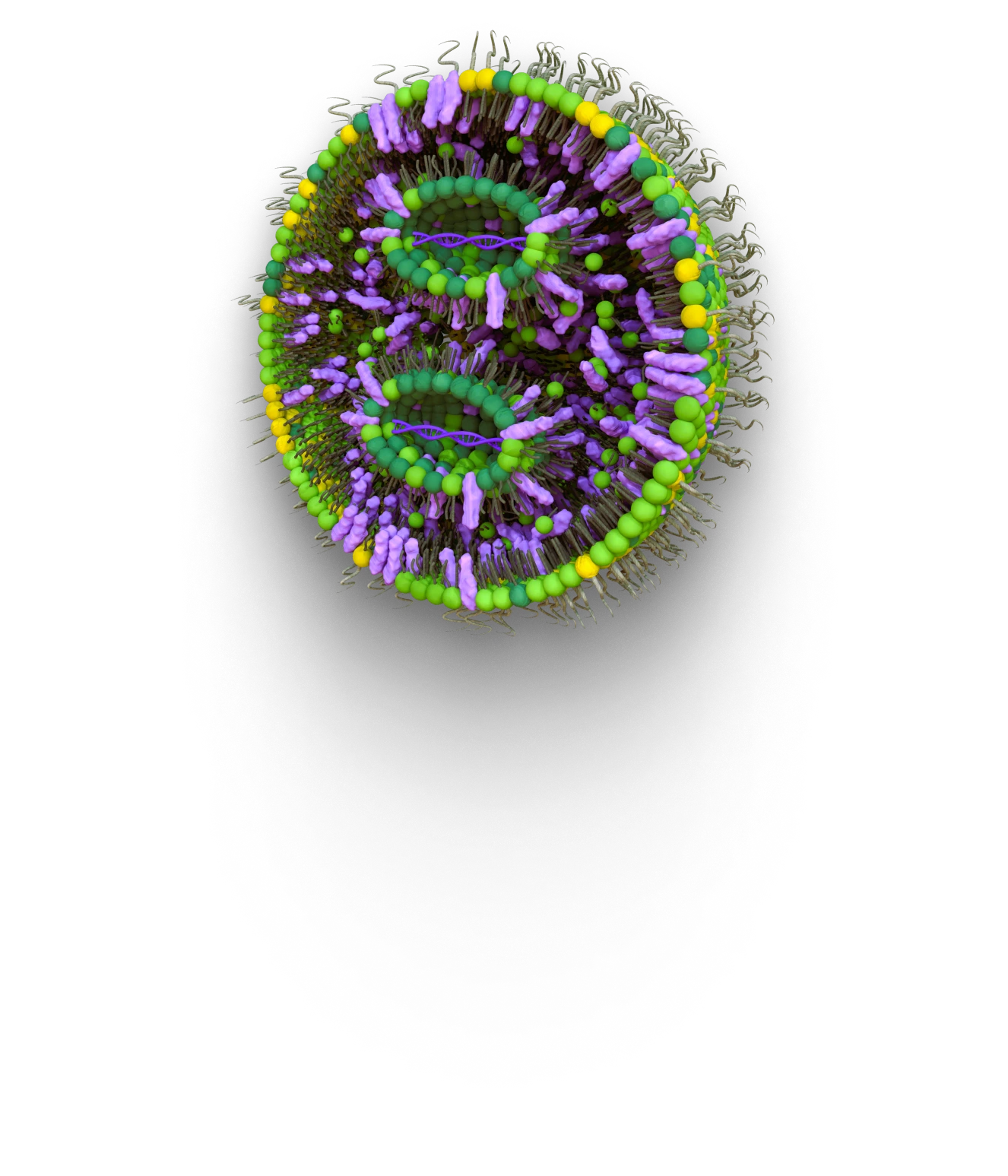
Transforming Patients Lives:
Complete Assistance Along Your Path to Market Authorization
Preclinical Development
We establish robust, scalable processes, implement analytical methods, and manufacture non-GMP pilot batches.
- Tech Transfer and GAP analysis Polymer and lipid excipients processes development
- Polymer and lipid nanoparticles screening formulation
- Proprietary technologies
- Method Development and Testing
- Pre-stability/Forced Degradation Studies
- Pilot Scale Manufacturing for non-clinical supply/GLP Tox studies
Clinical
Supply
We ensure seamless progress towards IND filing in novel functional excipient, drug substance and drug product. We support your project from candidate optimization to GLP tox studies towards GMP production.
- IND/IMPD Enabling Package
- Polymer and lipid excipients process development
- Method Validation and Process Characterization
- Clinical Trial Materials Supply
- ICH Stability Studies
- Regulatory Package
- Batch Certification and release by Qualified Person including investigational medicinal products
Commercial Manufacturing
We provide comprehensive services for commercial scale manufacturing, GMP compliance and regulatory processes to bring products to market in an agile and reliable way.
- Process and Method Validation
- Commercial Supply
- ICH Stability Studies
- Assistance for NDA or BLA regulatory approvals
Navigating the Complexities of Drug Development with Seasoned Expertise
Our expertise spans drug delivery systems, functional excipients and analytical methods. With a team of industry-leading subject matter experts, we provide our customers with the high-quality products they need to advance innovation in the cell and gene therapy space.

Our technical experts are specialists recognized worldwide in the following key areas:




Products Tailored to You
Our dedication to staying at the forefront of our industry means that our catalog products, including proprietary and reference items, are constantly updated to reflect the latest advancements and trends. This guarantees that our customers have access to the most cutting-edge solutions in non-viral drug delivery systems.

Explore Our Resources
As a global organization, we are actively participating in events and projects that are at the core of our field. Explore our technical library composed of technical application notes, brochures, and case studies to better understand our core capabilities, services, and technology.
Testimonials
At Curapath, customer satisfaction is our main target, and we deeply appreciate their valuable feedback. We are fully committed to delivering personalized support, high quality products and services. With a current Net Promoter Score (NPS) of 61, we proudly reflect a high level of customer satisfaction.

“At KNAUER Wissenschaftliche Geräte GmbH, we have had the privilege of collaborating with Curapath on multiple occasions. Through our professional experiences with them, we have consistently been impressed by their unwavering commitment to excellence, the expertise they bring to every project, and their genuine drive to push the boundaries of innovation.
We wholeheartedly recommend Curapath to any organization seeking top-tier solutions and an exemplary working relationship. Their integrity, expertise, and commitment to clients are unparalleled. We look forward to many more years of fruitful collaboration.”

“We only have a small project with Curapath, but communication from the BD team and project manager have always been impeccable. They are easy and quick to understand what I as a customer want and need, they come up with proposals and solutions, they are always responsive, super friendly, and well-prepared for our regular meetings – it’s a breeze and makes my life so much easier. The R&D team works diligently, with proper care of the product, and the holistic project strategy in mind, and wherever appropriate, make smart recommendations for strategy adaptations or additional tests to ensure maximum outcome for the customer.”

“We had the opportunity to work with the Curapath team for a while. It was really incredible how the team was focused on delivering results and solutions to us.
But more valuable than that was the way we managed to grow together and how they anticipated our requirements so that we consistently achieved the milestones of our projects in time and on budget. We all need a committed Partner like Curapath to achieve our goals and grow the business.”

“Curapath (formerly PTS) is an excellent CDMO with invaluable experience in synthetic biological polymers and nanoparticle formulation. We have worked closely with them and have been impressed with their technical expertise and customer-focused approach. Our interactions are consistently professional and beneficial.”

“We have found with Curapath a partner who has a profound know-how in both technical aspects of developing and manufacturing real innovative carriers in the field of polymer therapeutics and, furthermore, a deep understanding of the administrative and documentation necessities in the pharma world.
Both are extremely important and valuable, as new developments in the field of biopharmaceuticals require a solid technical understanding from chemistry to pharmacology to biochemistry and medicinal chemistry, as well as the special regulatory aspects, which have to be matched by any party acting in the health industry.”


“We are fortunate to have Curapath as our partners for the scale-up process of our adhesive hydrogel technology. They consistently provided high-quality materials and quickly adapted to new product requirements, all while maintaining the highest degree of quality and scientific rigor.”

“I have been working with Curapath for different companies on research and early-stage development projects ranging from screening of lipid-based formulations for nucleic acid delivery to developing manufacturing processes for protein-based drug carriers to perform IND enabling studies. Having worked with many CDMOs in the last 25 years, I was always impressed by their outstanding technical competence, their deep understanding of the challenges of drug development, and their responsiveness to the client’s needs.”

“I have been fortunate to work with Curapath on a number of projects where they have been an excellent partner as a CMO and fantastic team members in research and development activities.
Having worked within the polypeptide field for more than 25 years, I have seen and been involved with many efforts to commercialize these specialty polymers. In my view, Curapath has achieved a depth of understanding and a level of expertise in polypeptide manufacturing and analytics that is second to none.
I see Curapath as a tremendous asset…, and highly recommend them as a valuable resource for all polypeptide manufacturing needs.”

“Filling the gap between academia and industry is a big challenge. Curapath is an ideal partner for application-oriented academic research in order to transfer fundamental research into the market. The Curapath research team possesses a high level of training in scientific research combined with a privileged version of the social needs.”
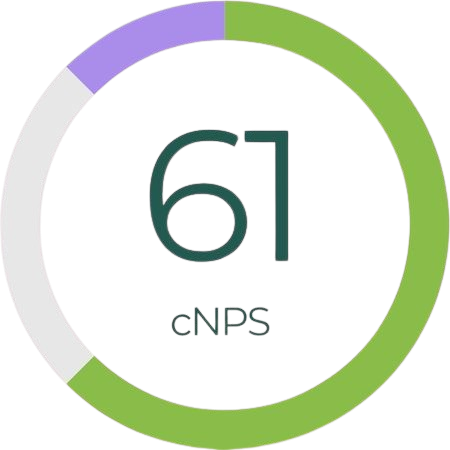
cNPS performed by external partner Hubspot in 2024

